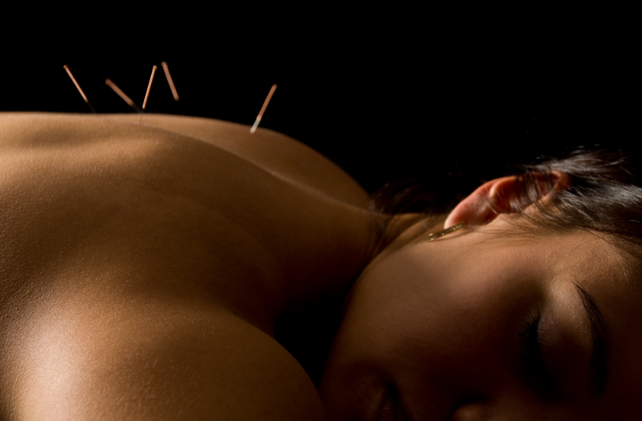
Acupuncture Provides Long Term Pain Relief for Joint Pain Caused by Aromatase Inhibitors
In a randomized study conducted in November 2022 by a research team headed by Dr. Dawn Hershman MD, asked
“Does short-term acupuncture confer long-term reduction of joint pain related to aromatase inhibitors among women with breast cancer?”
This study demonstrated the benefits and highlights the durability of response to Acupuncture which significantly relieved joint pain caused by aromatase inhibitors in women diagnosed with early-stage, hormone receptor-positive breast cancer for one year.
Patients received 18 acupuncture treatments over 12 weeks.
This is a typical and traditional course of acupuncture applied to achieve a real pattern change and durable outcome. Controls included an second set of patients who received sham acupuncture and a third group of patients were told their were on a waiting list and received no treatment. All patients had been receiving AI therapy for at least 30 days at inception.
Patients were monitored for another 40 weeks and thus were followed for a full year.
“The study was conducted at 11 academic and community sites within the National Cancer Institute Community Oncology Research Program. Sites were required to have 2 trained acupuncturists for the duration of the trial.” (1)
Aromatase Inhibitors (AI) are widely used in the treatment of estrogen receptor positive breast cancers. AI inhibit the transformation of androgens in the tissue into biologically active estrogens which can bind to the receptors on breast cancer cells sending a growth signal. Aromatase inhibitors are usually prescribed after surgery for five to ten years to reduce risk of recurrence in post-menopausal women.
Commonly used first line AI include Arimidex (anastrozole), Aromasin (exemestane) and Femara (letrozole).
However a common adverse effect is joint pain and stiffness which contributes to non-compliance with therapy for more than 50% of breast cancer patients. Many patients do not disclose to their physicians that they have discontinued AI therapy due to poor quality of life and persistent pain.
Researchers concluded that “Acupuncture was associated with a statistically significant decrease in aromatase inhibitor–related joint pain that persisted at 40 weeks after discontinuation of the intervention, suggesting long-term benefits of this therapy.” 
(1) The study showed that a full course of therapeutic acupuncture over three months led to a durable change in perceived pain at 52 weeks compared to controls. This study did not follow women past 52 weeks.
Subsequent systemic reviews and meta-analyses (2, 3) of acupuncture trials have also demonstrated efficacy and long term beneficial effect.
1.Hershman, D. Et al Comparison of Acupuncture vs Sham Acupuncture or Waiting List Control in the Treatment of Aromatase Inhibitor-Related Joint Pain: A Randomized Clinical Trial. JAMA Netw Open. 2022 Nov 1;5(11):e2241720. doi: 10.1001/jamanetworkopen.2022.41720. PMID: 36367721; PMCID: PMC9652759.
2. Liu X, Lu J, Wang G, et al. . Acupuncture for arthralgia induced by aromatase inhibitors in patients with breast cancer: a systematic review and meta-analysis. Integr Cancer Ther. 2021;20:1534735420980811. doi:10.1177/1534735420980811 - DOI - PMC - PubMed
3. MacPherson H, et al. ; Acupuncture Trialists’ Collaboration . The persistence of the effects of acupuncture after a course of treatment: a meta-analysis of patients with chronic pain. Pain. 2017;158(5):784-793. doi:10.1097/j.pain.0000000000000747 - DOI - PMC - PubMed