Xeloda (Capecitabine) is the oral form of chemotherapy agent 5-Fluorouracil (5-FU) which is administered intravenously.
Both drugs are widely used in a broad range of cancers. The mechanism of action is inhibition of mitosis (cell division).
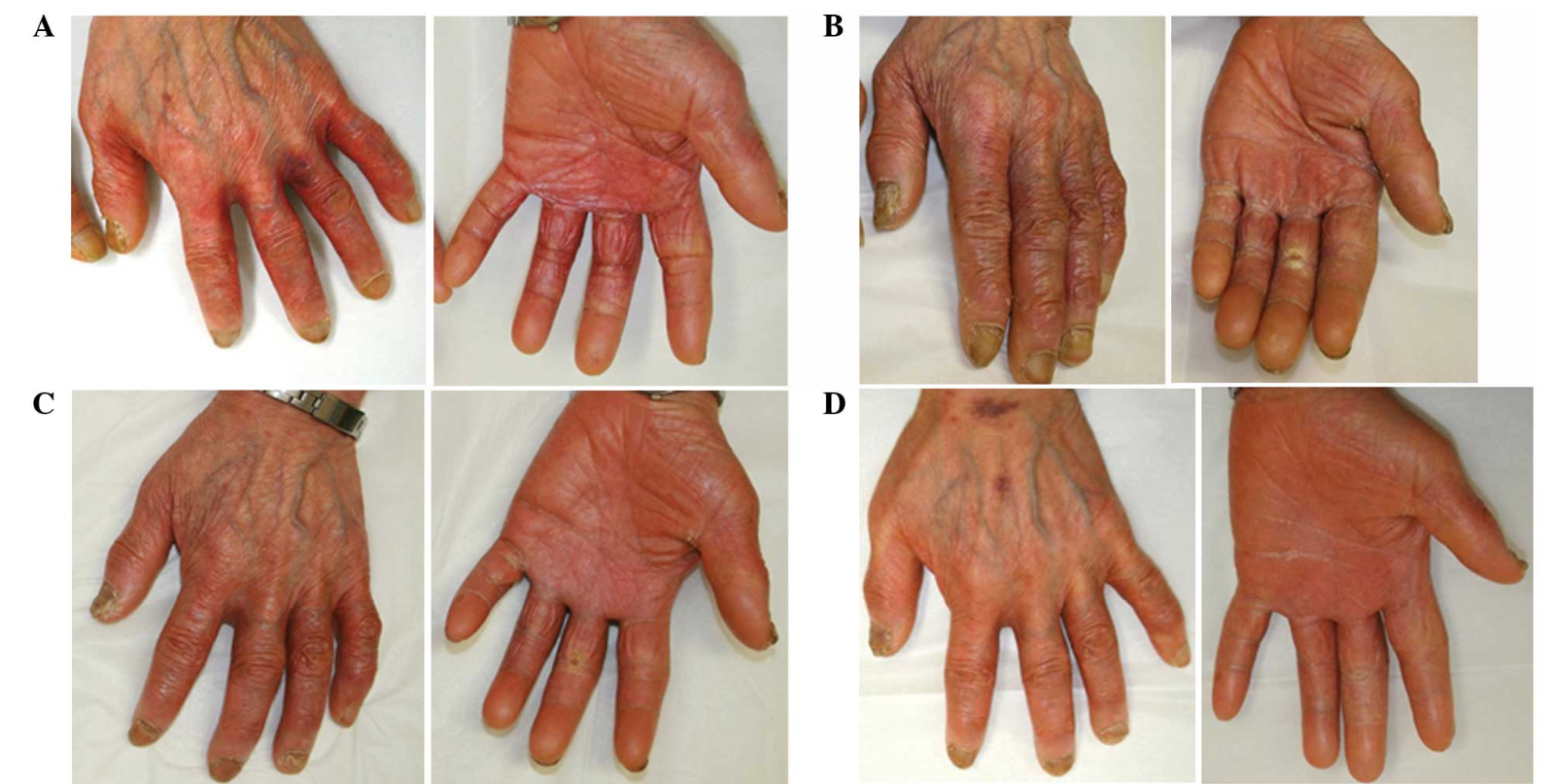
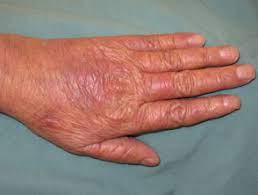
One of the most common side effects of these drugs is HAND-FOOT SYNDROME or palmar-plantar erythrodysesthesia. Hand-Foot syndrome presents with redness, swelling and pain, tingling, burning and sensitivity to touch on the palms of the hands and the soles of the feet. Severe cases may include cracked, flaking or peeling skin, painful blisters, ulcers or sores, severe pain and difficulty walking or using the hands.
While etiology is not certain it is hypothesized that these drugs cause capillary fragility and subsequent leakage of drugs into and damage of the surrounding tissue.
Other commonly used chemotherapy agents which result in Hand - Foot syndrome include 5-Fluorouracil, Capecitabine, Cytarabine, Docetaxel, Doxorubicin, Doxil and Paclitaxel. These drugs are widely used in Breast, Ovarian and Gastrointestinal Cancers.
Not all patients who receive these drugs develop Hand-Foot Syndrome. The symptoms typically appear with the first dose administration in 40-50 percent of patients at grade 2 or higher.
Grade 1: painless erythema or dysesthesia, no impairment
Grade 2: painful erythema, swelling, tingling, numbness, dryness, cracking, Desquamation, activity is impaired
Grade 3: strong pain, ulceration, blistering, erythema, limited self-sufficiency
For drugs administered via IV infusion, cold (Ice) gloves and booties that constrict capillaries reduce local exposures in the hands and feet and must be worn during infusions and for several therapy to hands and feet concurrently with chemotherapy infusions.
Capcetabine increases COX-2 expression in both tumors and healthy tissues.
A double blind placebo controlled study in which oral COX2 inhibitor celecoxib was administered to patients over a 2 year period while receiving capecitabine or capecitabine plus oxaliplatin demonstrated a significant decrease in the incidence and intensity of symptoms.
The phytochemical curcumin derived from Rhizoma Curcuma longa interacts with over 100 genes that impact tumor cell behavior and metabolism. Curcumin is known to decrease COX2 expression along with expression of pro-inflammatory NFkB, TNFa, IL1B, IL6 and IL8.
 In a human study researchers administered turmeric at a dose of 4 g/day (2 pills 12 hours apart) starting at the beginning of capecitabine treatment and lasting six weeks. The study included 40 patients whose mean age was 62 years. Most were female (80%), 52% had breast cancer, and 47.5% had GI tumors. After the first cycle of capecitabine treatment, 11 of 40 patients developed HFS (27.5%; 95% CI [15, 42]), whereas four patients developed HFS equal or superior to grade 2 (10%; 95% CI [3.3, 23]).. The study concluded that turmeric combined with capecitabine seems to produce a lower rate of HFS, especially grade 2 or higher.
In a human study researchers administered turmeric at a dose of 4 g/day (2 pills 12 hours apart) starting at the beginning of capecitabine treatment and lasting six weeks. The study included 40 patients whose mean age was 62 years. Most were female (80%), 52% had breast cancer, and 47.5% had GI tumors. After the first cycle of capecitabine treatment, 11 of 40 patients developed HFS (27.5%; 95% CI [15, 42]), whereas four patients developed HFS equal or superior to grade 2 (10%; 95% CI [3.3, 23]).. The study concluded that turmeric combined with capecitabine seems to produce a lower rate of HFS, especially grade 2 or higher.
Comments
- There were no contra-indications to utilizing turmeric concurrently with capecitabine
- A significant therapeutic dose of 4 grams per day was used. In my practice I prefer to use the more concentrated isolate curcumin and dose at 2-4 grams per day. This would yield more inflammation control.
- Turmeric falls into the category of herbs that “Move Stagnant Blood” in Chinese Medicine. One of the properties of curcumin is inhibition of platelet aggregation.
References:
Curcuma longa (Turmeric) for Prevention of Capecitabine-Induced Hand-Foot Syndrome: A Pilot Study
Vanessa Armenio Scontre , MD,Janine Capobiango Martins , MD, et al
Jnl Diet Supp Pages 606-612 | Published online: 02 Nov 2017
https://doi.org/10.1080/19390211.2017.1366387 PMID: 2909565
The effect of COX-2 inhibitor on capecitabine-induced hand-foot syndrome in patients with stage II/III colorectal cancer: a phase II randomized prospective study.Zhang RX, Wu XJ, Lu SX, Pan ZZ, Wan DS, Chen G.
J Cancer Res Clin Oncol. 2011 Jun;137(6):953-7. doi: 10.1007/s00432-010-0958-9. Epub 2010 Nov 27.
PMID: 21113620 Clinical Trial.